Effective: 06-04-21
Supersedes: Unprotected Exposure/ Infection Control
PURPOSE
Brewster and EasCare Ambulance Service is committed to providing a safe and healthy work environment for our entire staff. In pursuit of this endeavor, the following exposure control plan (ECP) is provided to eliminate or minimize occupational exposure to bloodborne pathogens in accordance with OSHA standard 29 CFR 1910.1030, "Occupational Exposure to Bloodborne Pathogens."
This plan identifies and defines:
· General hazards associated with exposure to blood or to other potentially infectious materials;
· Specific tasks considered presenting a potential exposure to these hazards;
· Personal protective equipment and safe work practices designed to prevent exposure;
· Vaccination requirements;
· Exposure determination and follow up to include record keeping requirements.
POLICY
It is the intent of this plan to provide procedures which, when followed, will help prevent employee exposure to bloodborne infectious diseases. Company personnel shall take reasonable precautions to keep from being exposed to bloodborne pathogens and/or from acquiring infectious or communicable diseases from patients. Patients shall also be reasonably protected from acquiring infections while in our care.
Plan Maintenance
The Director of Policy and Regulatory Affairs, along with the Designated Infection Control Officer (DICO) is the custodian of the Infection Control Plan, and responsible for its maintenance. The plan shall be reviewed at least annually and updated as necessary to reflect new or modified tasks and procedures that may affect potential occupational exposure.
Definitions
Blood includes: Human blood. Animal blood is not included.
Bloodborne Body Fluids: include amniotic fluid, semen, vaginal fluids, and fluids that surround body organs. Human Bites: Saliva can be infectious for Hepatitis-B/C.
Decontamination: The use of physical or chemical means to remove, inactivate, or destroy bloodborne pathogens on a surface or item to the point where they are no longer capable of transmitting infectious particles and the surface or item is rendered safe for handling,use, or disposal.
Exposure Incident: means a specific eye, mouth, other mucous membrane, non-intact skin, or parenteral contact with blood or other potentially infectious materials that results from the performance of an employee's duties
Other Potentially Infectious Materials: These include the following human body fluids: semen, vaginal secretions, cerebrospinal fluid, synovial fluid, peritoneal fluid, amniotic fluid, pleural fluid, saliva where there has been mouth trauma, any bodily fluid that is visibly contaminated with blood and all body fluids in situations where it is difficult or impossible to differentiate between body fluids.
Exposure Determination
Emergency Medical Technicians (Basic and Advanced), Paramedics, Field Supervisors and, to a lesser extent, chair car operators may be exposed to blood or other potentially infectious materials. These potential exposures may occur during:
· Trauma treatment (bleeding control, wound care,etc.)
· Crime scene with blood
· Handling syringes used by the public, or during the course of providing treatment
· Decontamination of surfaces contaminated with blood or other potentially infectious materials.
· Caring for patients who may be assaultive and bite
· Intravenous cannulation
· Inserting oral airways, suctioning, or intubating
· Assisting with childbirth
· Administering aerosol generating procedures
Universal Precautions
All human blood, body fluids and other potentially infectious materials as defined in 29 CFR 1910.1030 shall be treated as if known to be infectious for HIV, Hepatitis, and other bloodborne pathogens. EMS personnel should consider all patients as potential carriers of infectious disease(s) and observe appropriate Universal Blood and Body Secretion Precautions.
Engineering Controls
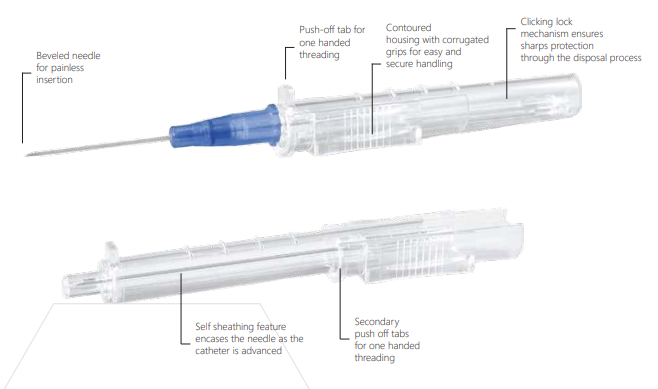
· The company utilizes sharps designed with built-in safety features or mechanisms that effectively reduces the risk of an exposure incident. Intravenous catheters are designed with a clicking lock mechanism to ensure safety from cross contamination. Whenever possible, intravenous medications are administered via luer lock connections to reduce the use of needles, and IM/SC injection needles are designed to retract or have hinged needle covers to facilitate easier one-hand application of the needle sheath.

· All ambulances and support vehicles are supplied with needle boxes made out of hard plastic. The needle boxes are secured either by an adhesive pad or within a bracket mounted near the point of use. Specimens of blood or other potentially infectious materials shall be placed in a container that prevents leakage during collection, handling, processing, storage, transport, or shipping.
· Ventilators and bag valve masks are equipped with bacterial / viral filters
Work Practice Controls
Work practice controls shall be adopted to complement adopted engineering controls.
· Personnel shall wash all potentially exposed skin with soap and water; or flush mucous membranes with water immediately or as soon as feasible following contact of such body areas with blood or other potentially infectious materials. If hand washing facilities are not immediately available, personnel are to wipe exposed skin with the germicidal solution located in each vehicle. Hand washing shall occur at the earliest opportunity.
· Contaminated needles and other sharps shall not be recapped, bent or removed. Instead, they are to be placed in an appropriate needle box container as soon as possible. Red biohazard plastic bags will be stored on each ambulance for the temporary storage of contaminated material. Upon arrival at receiving hospital or back at the station, the biohazard bags will be transferred to the primary disposal container located in the Decontamination Area.
· Eating, smoking, applying lip balm or any cosmetics, and handling contact lenses is prohibited in the patient care area of any ambulance.
· Food, cosmetics, and tobacco products shall not be stored in the patient care area of an ambulance or in the Decontamination Area at a station.
· All procedures involving blood or other potentially infectious materials shall be performed in such a manner as to minimize splashing, spraying, spattering, and generation of droplets of these substances.
Personal Protective Equipment
Where potential occupational exposure exists, personal protective equipment (PPE) shall be utilized. The following PPE is located on all ambulances and is readily available for personnel:
· Disposable gloves
· Impervious gowns
· Eye shields
· Surgical masks
· Resuscitation bags
· Type N-95 respirator masks
PPE should be removed before leaving the work area. Disposable gloves shall be replaced as soon as practical when contaminated or when they are torn, punctured, or when their ability to function as a barrier is compromised. Gloves should be changed between patients when practical, and discarded before entering the cab of the ambulance.
Masks and protective eyewear or face shields shall be worn in the patient care compartment and when working within six feet of a patient who is suspected of having a disease transmitted by droplets. They shall also be worn during aerosol generating procedures such as nebulizer treatments, or other procedures that are likely to generate droplets or a spray of blood or release of other body fluids to prevent exposure of mucous membranes of the mouth, nose and eyes. During times of pandemic or new disease emergence, personnel may be directed to increase respiratory protection in accordance with the Office of Emergency Medical Services and/or CDC guidance.
Gowns and other protective body clothing shall be worn in occupational exposure situations to the degree determined by the task and degree of exposure anticipated.
Hand Washing
Handwashing is required at the start of the shift, after using toilet facilities, after each patient contact, and after vehicle or equipment cleaning or maintenance. An alcohol-based solution for hand sanitizing is available on each vehicle and shall be used after patient contact or contact with blood/body fluids when running water and soap are not available. When decontaminating hands with an alcohol-based hand rub, apply product to palm of one hand and rub hands together, covering all surfaces of fingers and fingernails until hands are dry. Follow the manufacturer’s recommendations regarding the volume of product to use.
The handwashing procedure should be repeated with soap and running water as soon as possible. When washing hands with soap and water, wet hands first with warm water, apply an amount of product recommended by the manufacturer to hands and wrists and rub together vigorously for at least 20-30 seconds, covering all surfaces and the hands and fingers. Rinse hands with water and dry thoroughly with a disposable towel. Use a clean, dry (new) towel to turn off the faucet. Avoid using hot water, because repeated exposure to hot water may increase the risk of dermatitis.
Routine Cleaning and Disinfection
All equipment and areas of the patient compartment that may have come in contact with a patient’s blood or bodily fluids such as the walls, floors, seats, grab rails, stretchers, etc., shall be wiped clean of obvious debris and decontaminated. The product selected should have a product label that shows that the product has been tested against HIV, Hepatitis B and TB. Team members shall wear gloves while decontaminating equipment. Fluid shield masks and gowns shall be used if splashing is anticipated. Equipment shall only be decontaminated in areas that are designated as appropriate by the facility and/or staff.
Regulated Waste
Infectious / regulated waste will be disposed of in accordance with applicable Department of Public Health regulation and OSHA standard on bloodborne pathogens. The following materials must be disposed of in an approved Sharps disposal container:
· Syringes
· Lancets
· Intravenous catheters
· Epi-Pen or other auto-injectors
· Used razors
The following materials can be disposed of in regular trash:
· Paper towels, rags or other items that are contaminated with blood, but blood cannot be squeezed out of the fabric.
· Used band-aids and gauze when blood cannot be squeezed out of the fabric
· Feminine hygiene products
The following materials should be disposed of in a plastic “red bag” that is labelled as medical waste:
· Paper towels, rags, or clothing items that are contaminated with copious amounts of blood in which blood is dripping out of the fabric.
· Used gloves when blood is dripping out of fabric or material.
When moving containers of contaminated sharps from the area, the containers shall be closed immediately prior to removal or replacement to prevent spillage or protrusion of contents during handling, storage, transport, or shipping. The containers shall be placed in a secondary container if leakage is possible. The secondary container shall be closable, constructed to maintain all contents and prevent leakage during handling, storage, transport, or shipping and color-coded / labelled in accordance with OSHA standard.
Laundry
Contaminated laundry should be handled as little as possible with a minimum of agitation. Clothing and linen that is contaminated with blood may be laundered:
· Place contaminated laundry into a plastic bag, label as contaminated.
· Put laundry into washing machine, directly out of the plastic bag without sorting. Don healthcare gloves (neoprene, nitrile) to put clothing into the machine.
· Use hot water wash.
· Add ¼ cup bleach using machine instructions.
Vaccination
The Hepatitis-B vaccine is very effective in preventing transmission of the Hepatitis-B virus. Company personnel should be vaccinated as follows, depending upon their potential exposure to blood and body fluids:
· Employees who perform tasks with potential exposure to blood or body fluids are offered Hepatits-B vaccine at time of hire. Employees where exposure to blood or body fluids is not expected (communication center, billing/administrative, fleet services) will be offered the vaccine in the event of a workplace exposure.
· The vaccine consists of three doses administered at 1-week, 1-month, and 6-month intervals. The vaccine also includes an antibody test to see of the vaccine was effective. The antibody test should be conducted at 4-8 weeks after the third dose.
· Employees who decline the vaccine will sign a waiver.
· Employees who initially decline the vaccine but who later wish to have it may then have the vaccine provided at no cost.
Unprotected Exposure / DICO
An exposure incident is defined as a specific eye, mouth, or other mucous membrane, non-intact skin, or parenteral contact with blood or other potentially infectious materials that results from the performance of a person’s duties.
All personnel who believe they have experienced an exposure event should first provide themselves with the appropriate first-aid treatment and decontamination as required. Once able, personnel should contact a supervisor and the Designated Infection Control Officer (DICO) for guidance. [email:DICO@BrewsterAmbulance.com ] In addition to evaluating possible employee exposures to communicable diseases, the DICO coordinates communications between the company, area hospitals, Board(s) of Health, and other health care professionals. The DICO receives notifications of exposures to infectious diseases dangerous to the public health from health care facilities, and notifies the involved care providers of a potential exposure to an infectious disease dangerous to public health.
The Supervisor and DICO will evaluate the facts of the potential exposure and determine if there is a potential for occupational acquisition of an infectious disease, based on CDC Guidelines for Risk of Occupational Exposure and Recommendations for Post-Exposure prophylaxis. If it is determined that an exposure incident occurred, the team member will be directed to the appropriate facility (emergency department or Convenient MD) for treatment.
It is imperative that personnel report any potential exposure incident to the receiving facility and Field Supervisor. If you experience an Exposure Incident, get medical treatment within the first 24 hours. Studies have shown that receiving medical treatment for a needlestick within the first 24 hours is able to prevent transmission of HIV and Hepatitis.
1. Complete an unprotected exposure form and an incident report.
2. The form must be provided to the receiving facility upon arrival.
3. It is the responsibility of the receiving facility to evaluate the information reported, if the patient is diagnosed as having an infectious disease covered by the regulations.
4. If it is determined that you have sustained an unprotected exposure, it is the responsibility of the receiving facility to notify you orally within 48 hours and in writing within 72 hours from the time of the patient diagnosis. The receiving facility will also provide you with instructions concerning appropriate medical precautions and actions.
Source Testing: When the identity of the source person is known, he or she may be contacted to inform them that a public safety employee was exposed to the person’s blood, and request consent for the person to obtain testing for HIV, Hepatitis B and Hepatitis C. The results are not released to the employer--- the results should be maintained privately between the source person’s healthcare provider, the source person, and the exposed employee. If consent is not obtained from the source of the exposure, it shall be documented that consent cannot be obtained.
Sharps Injury Log
The Risk/Safety Department will maintain a sharps injury log for the recording of percutaneous injuries from contaminated sharps. The information in the sharps injury log shall be recorded and maintained in such a manner as to protect the confidentiality of the injured employee. The sharps injury log shall contain a minimum of:
· The date of the injury
· Type and brand of device involved (syringe, angiocath,etc)
· Department or Work area where the exposure incident occurred
· Explanation of how the incident occurred
Training
Training for all employees who are reasonably anticipated to have occupational exposure to blood and OPIM will be conducted prior to the initial assignment and annually. Training records will be maintained for three years from the date on which the training occurred. Training will include:
· The OSHA Bloodborne Pathogens Standard;
· Symptoms and transmission of bloodborne diseases;
· The employer’s exposure control plan, i.e., points of the plan, lines of responsibility, how the plan will be implemented, etc.;
· Control measures;
· Personal protective equipment and an explanation of the basis for its selection and use;
· Post-exposure follow/up and evaluation;
· Hepatitis B vaccine program.